- Tlc Plate Drawing Tool Free
- Tlc Plate Drawing Tools
- Tlc Plate Drawing Tool Online
- Tlc Plate Drawing Tool
TLC is a simple, quick, and inexpensive procedure that gives the chemist a quick answer as to how many components are in a mixture. TLC is also used to support the identity of a compound in a mixture when the Rf of a compound is compared with the Rf of a known compound (preferably both run on the same TLC plate).
A TLC plate is a sheet of glass, metal, or plastic which is coated with a thin layer of a solid adsorbent (usually silica or alumina). A small amount of the mixture to be analyzed is spotted near the bottom of this plate. The TLC plate is then placed in a shallow pool of a solvent in a developing chamber so that only the very bottom of the plate is in the liquid. This liquid, or the eluent, is the mobile phase, and it slowly rises up the TLC plate by capillary action.
As the solvent moves past the spot that was applied, an equilibrium is established for each component of the mixture between the molecules of that component which are adsorbed on the solid and the molecules which are in solution. In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will be carried farther up the plate than others. When the solvent has reached the top of the plate, the plate is removed from the developing chamber, dried, and the separated components of the mixture are visualized. If the compounds are colored, visualization is straightforward. Usually the compounds are not colored, so a UV lamp is used to visualize the plates. (The plate itself contains a fluorescent dye which glows everywhere except where an organic compound is on the plate.)
How To Run a TLC Plate
Step 1: Prepare the developing containerThe developing container for TLC can be a specially designed chamber, a jar with a lid, or a beaker with a watch glass on the top (the latter is used in the undergrad labs at CU). Pour solvent into the chamber to a depth of just less than 0.5 cm. To aid in the saturation of the TLC chamber with solvent vapors, you can line part of the inside of the beaker with filter paper. Cover the beaker with a watch glass, swirl it gently, and allow it to stand while you prepare your TLC plate. |
Step 2: Prepare the TLC plateTLC plates used in the organic chem teaching labs are purchased as 5 cm x 20 cm sheets. Each large sheet is cut horizontally into plates which are 5 cm tall by various widths; the more samples you plan to run on a plate, the wider it needs to be. Shown in the photo to the left is a box of TLC plates, a large un-cut TLC sheet, and a small TLC plate which has been cut to a convenient size. Handle the plates carefully so that you do not disturb the coating of adsorbent or get them dirty. |
| Measure 0.5 cm from the bottom of the plate. Using a pencil, draw a line across the plate at the 0.5 cm mark. This is the origin: the line on which you will spot the plate. Take care not to press so hard with the pencil that you disturb the adsorbent. Under the line, mark lightly the name of the samples you will spot on the plate, or mark numbers for time points. Leave enough space between the samples so that they do not run together; about 4 samples on a 5 cm wide plate is advised. |
Step 3: Spot the TLC plateIf the sample is not already in solution, dissolve about 1 mg in 1 mL of a volatile solvent such as hexanes, ethyl acetate, or methylene chloride. As a rule of thumb, a concentration of 1% usually works well for TLC analysis. If the sample is too concentrated, it will run as a smear or streak (see troubleshooting section below); if it is not concentrated enough, you will see nothing on the plate. Sometimes you will need to use trial and error to get well-sized, easy to read spots. |
| Obtain a a microcapillary. In the organic teaching labs, we use 10µL microcaps - they are easier to handle than the smaller ones used in research labs. Dip the microcap into the solution and then gently touch the end of it onto the proper location on the TLC plate. Don't allow the spot to become too large - if necessary, you can touch it to the plate, lift it off and blow on the spot. If you repeat these steps, the wet area on the plate will stay small. |
| This example plate has been spotted with three different quantities of the same solution and is ready to develop. If you are unsure of how much sample to spot, you can always spot multiple quantities and see which looks best. |
Step 4: Develop the platePlace the prepared TLC plate in the developing beaker, cover the beaker with the watch glass, and leave it undisturbed on your bench top. The solvent will rise up the TLC plate by capillary action. Make sure the solvent does not cover the spot. |
| Allow the plate to develop until the solvent is about half a centimeter below the top of the plate. Remove the plate from the beaker and immediately mark the solvent front with a pencil. Allow the plate to dry. |
Step 5: Visualize the spotsIf there are any colored spots, circle them lightly with a pencil. Most samples are not colored and need to be visualized with a UV lamp. Hold a UV lamp over the plate and circle any spots you see. Beware! UV light is damaging both to your eyes and to your skin! Make sure you are wearing your goggles and do not look directly into the lamp. Protect your skin by wearing gloves. |
| If the TLC plate runs samples which are too concentrated, the spots will be streaked and/or run together. If this happens, you will have to start over with a more dilute sample to spot and run on a TLC plate. |
| Here's what overloaded plates look like compared to well-spotted plates. The plate on the left has a large yellow smear; this smear contains the same two compounds which are nicely resolved on the plate next to it. |
TLC Solvents Choice
Preparing the Plate Do not touch the TLC plate on the side with the white surface. In order to obtain an imaginary start line, make two notches on each side of the TLC plate. You can also draw a thin line with pencil. The start line should be 0.5-1 cm from the bottom of the plate. Usually, a thin layer chromatography plate is around 5–7 cm high, and a line is drawn around 0.5–1.0 cm from the bottom. That is the line in which you will spot your mixtures to separate. Download ultraman fighting evolution 3 pcsx2 games for pc. It is important that you spot the mixtures above the solvent level on your elution chamber!
When you need to determine the best solvent or mixture of solvents (a 'solvent system') to develop a TLC plate or chromatography column loaded with an unknown mixture, vary the polarity of the solvent in several trial runs: a process of trial and error. Carefully observe and record the results of the chromatography in each solvent system. You will find that as you increase the polarity of the solvent system, all the components of the mixture move faster (and vice versa with lowering the polarity). The ideal solvent system is simply the system that gives the best separation.
TLC elution patterns usually carry over to column chromatography elution patterns. Since TLC is a much faster procedure than column chromatography, TLC is often used to determine the best solvent system for column chromatography. For instance, in determining the solvent system for a flash chromatography procedure, the ideal system is the one that moves the desired component of the mixture to a TLC Rf of 0.25-0.35 and will separate this component from its nearest neighbor by difference in TLC Rf values of at least 0.20. Therefore a mixture is analyzed by TLC to determine the ideal solvent(s) for a flash chromatography procedure.
Beginners often do not know where to start: What solvents should they pull off the shelf to use to elute a TLC plate? Because of toxicity, cost, and flammability concerns, the common solvents are hexanes (or petroleum ethers/ligroin) and ethyl acetate (an ester). Diethyl ether can be used, but it is very flammable and volatile. Alcohols (methanol, ethanol) can be used. Acetic acid (a carboxylic acid) can be used, usually as a small percentage component of the system, since it is corrosive, non-volatile, very polar, and has irritating vapors. Acetone (a ketone) can be used. Methylene chloride or and chloroform (halogenated hydrocarbons) are good solvents, but are toxic and should be avoided whenever possible. If two solvents are equal in performance and toxicity, the more volatile solvent is preferred in chromatography because it will be easier to remove from the desired compound after isolation from a column chromatography procedure.
Ask the lab instructor what solvents are available and advisable. Then, mix a non-polar solvent (hexanes, a mixture of 6-carbon alkanes) with a polar solvent (ethyl acetate or acetone) in varying percent combinations to make solvent systems of greater and lesser polarity. The charts below should help you in your solvent selection. You can also download this pdf chart of elution order.
Interactions Between the Compound and the Adsorbent
The strength with which an organic compound binds to an adsorbent depends on the strength of the following types of interactions: ion-dipole, dipole-dipole, hydrogen bonding, dipole induced dipole, and van der Waals forces. With silica gel, the dominant interactive forces between the adsorbent and the materials to be separated are of the dipole-dipole type. Highly polar molecules interact fairly strongly with the polar SiOH groups at the surface of these adsorbents, and will tend to stick or adsorb onto the fine particles of the adsorbent while weakly polar molecules are held less tightly. Weakly polar molecules generally tend to move through the adsorbent more rapidly than the polar species. Roughly, the compounds follow the elution order given above.
The Rf value
The retention factor, or Rf, is defined as the distance traveled by the compound divided by the distance traveled by the solvent.
For example, if a compound travels 2.1 cm and the solvent front travels 2.8 cm, the Rf is 0.75:
The Rf for a compound is a constant from one experiment to the next only if the chromatography conditions below are also constant:
- solvent system
- adsorbent
- thickness of the adsorbent
- amount of material spotted
- temperature
Since these factors are difficult to keep constant from experiment to experiment, relative Rf values are generally considered. 'Relative Rf' means that the values are reported relative to a standard, or it means that you compare the Rf values of compounds run on the same plate at the same time.
The larger an Rf of a compound, the larger the distance it travels on the TLC plate. When comparing two different compounds run under identical chromatography conditions, the compound with the larger Rf is less polar because it interacts less strongly with the polar adsorbent on the TLC plate. Conversely, if you know the structures of the compounds in a mixture, you can predict that a compound of low polarity will have a larger Rf value than a polar compound run on the same plate.
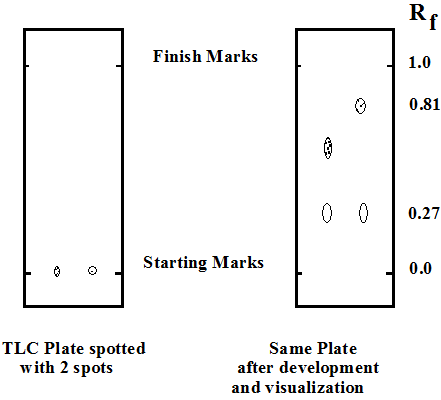
The Rf can provide corroborative evidence as to the identity of a compound. If the identity of a compound is suspected but not yet proven, an authentic sample of the compound, or standard, is spotted and run on a TLC plate side by side (or on top of each other) with the compound in question. If two substances have the same Rf value, they are likely (but not necessarily) the same compound. If they have different Rf values, they are definitely different compounds. Note that this identity check must be performed on a single plate, because it is difficult to duplicate all the factors which influence Rf exactly from experiment to experiment.
Troubleshooting TLC
All of the above (including the procedure page) might sound like TLC is quite an easy procedure. But what about the first time you run a TLC, and see spots everywhere and blurred, streaked spots? As with any technique, with practice you get better. Examples of common problems encountered in TLC:
- The compound runs as a streak rather than a spot: The sample was overloaded. Run the TLC again after diluting your sample. Or, your sample might just contain many components, creating many spots which run together and appear as a streak. Perhaps, the experiment did not go as well as expected.
- The sample runs as a smear or a upward crescent: Compounds which possess strongly acidic or basic groups (amines or carboxylic acids) sometimes show up on a TLC plate with this behavior. Add a few drops of ammonium hydroxide (amines) or acetic acid (carboxylic acids) to the eluting solvent to obtain clearer plates.
- The sample runs as a downward crescent: Likely, the adsorbent was disturbed during the spotting, causing the crescent shape.
- The plate solvent front runs crookedly: Either the adsorbent has flaked off the sides of the plate or the sides of the plate are touching the sides of the container (or the paper used to saturate the container) as the plate develops. Crooked plates make it harder to measure Rf values accurately.
- Many random spots are seen on the plate: Make sure that you do not accidentally drop any organic compound on the plate. If get a TLC plate and leave it laying on your workbench as you do the experiment, you might drop or splash an organic compound on the plate.
- You see a blur of blue spots on the plate as it develops: Perhaps you used an ink pen instead of a pencil to mark the origin?
- No spots are seen on the plate: You might not have spotted enough compound, perhaps because the solution of the compound is too dilute. Try concentrating the solution, or spot it several times in one place, allowing the solvent to dry between applications. Some compounds do not show up under UV light; try another method of visualizing the plate (such as staining or exposing to iodine vapor). Or, perhaps you do not have any compound because your experiment did not go as well as planned. If the solvent level in the developing jar is deeper than the origin (spotting line) of the TLC plate, the solvent will dissolve the compounds into the solvent reservoir instead of allowing them to move up the plate by capillary action. Thus, you will not see spots after the plate is developed. These photos show how the yellow compound is running into the solvent when lifted from the developing jar.
TLC Technique Quiz
See how well you understand TLC by taking the online TLC Technique Quiz!
Introduction
Thin-layer chromatography (TLC) is a very commonly used technique in synthetic chemistry for identifying compounds, determining their purity and following the progress of a reaction. It also permits the optimization of the solvent system for a given separation problem. In comparison with column chromatography, it only requires small quantities of the compound (~ng) and is much faster as well.
Stationary Phase
As stationary phase, a special finely ground matrix (silica gel, alumina, or similar material) is coated on a glass plate, a metal or a plastic film as a thin layer (~0.25 mm). In addition a binder like gypsum is mixed into the stationary phase to make it stick better to the slide. In many cases, a fluorescent powder is mixed into the stationary phase to simplify the visualization later on (e.g. bright green when you expose it to 254 nm UV light).
Preparing the Plate
Do not touch the TLC plate on the side with the white surface. In order to obtain an imaginary start line, make two notches on each side of the TLC plate. You can also draw a thin line with pencil. Do not use pen. Why? The start line should be 0.5-1 cm from the bottom of the plate.
Capillary spotters
Place a melting point capillary and in the dark blue part of the Bunsen burner flame. Hold it there until it softens and starts to sag. Quickly remove the capillary from the flame and pull on both ends to about 2-3 times its original length. If you pull the capillary inside the flame, you will have a 'piece of art', but not a good spotter. Allow the capillary to cool down, and then break it in the middle. Make sure that you break off the closed end on one of them. Do not use gloves when you pull capillaries. You will have much better control without them!
Tlc Plate Drawing Tool Free
Watch movie how to pull capillaries here here
Spotting the plate
Tlc Plate Drawing Tools
The thin end of the spotter is placed in the dilute solution; the solution will rise up in the capillary (capillary forces). Touch the plate briefly at the start line. Allow the solvent to evaporate and spot at the same place again. This way you will get a concentrated and small spot. Try to avoid spotting too much material, because this will deteriorate the quality of the separation considerably (‘tailing’). The spots should be far enough away from the edges and from each other as well. If possible, you should spot the compound or mixture together with the starting materials and possible intermediates on the plate. They will serve as internal reference since every TLC plate is slightly different.
Developing a Plate
A TLC plate can be developed in a beaker or closed jar (see picture below). Place a small amount of solvent (= mobile phase) in the container. The solvent level has to be below the starting line of the TLC, otherwise the spots will dissolve away. The lower edge of the plate is then dipped in a solvent. The solvent (eluent) travels up the matrix by capillarity, moving the components of the samples at various rates because of their different degrees of interaction with the matrix (=stationary phase) and solubility in the developing solvent. Non-polar solvents will force non-polar compounds to the top of the plate, because the compounds dissolve well and do not interact with the polar stationary phase. Allow the solvent to travel up the plate until ~1 cm from the top. Take the plate out and mark the solvent front immediately. Do not allow the solvent to run over the edge of the plate. Next, let the solvent evaporate completely.
TLC chamber for development e.g. beacher with a lid or a closed jar | after ~5 min | after ~10 min | after drying |
Visualization
Reagent | Works well for | Colors | Notes |
Iodine | Unsaturated and aromatic compounds | Brown spots | Not permanent |
Sulfuric acid | General stain | Brown or black spots | |
Chromic acid | For difficult to stain compounds | Black spots | |
UV light | Compounds with extended conjugation like aromatic compounds | Pink on light green background | Only visible under UV light |
Cerium sulfate | Good general stain, very well for alkaloids | ||
Ferric chloride | Phenols | Purple | |
Ninhydrin | Amino acids, amines | Purple | |
2,4-Dinitrophenylhydrazine | Aldehydes, ketones | Yellow/orange | also called “DNP” |
Vanillin | Good general stain, very well for hydroxyl or carbonyl compounds | Colors vary | |
Potassium permanganate | Works well for all compounds that can be oxidized | Yellow on purple
| at r.t. for alkenes and alkynes upon heating for alcohols, amines, sulfides |
Bromocresol Green | Carboxylic acids (pKa<5) | Yellow spot on blue background | |
Cerium molybdate (CAM, ‘Hanessian’s Stain’, Ceric staining) | Good general stain, very well with polyhydroxylated and carbonyl compounds | Blue or green spot | Upon heating, very sensitive! |
p-Anisaldehyde | Good general stain, particularly sensitive towards nucleophiles | Varying colors on light pink plate upon heating Autocad 2014 free student download. | Does not work with alkenes, alkynes or aromatic system unless functional groups are present |
Phosphomolybdic acid (PMA) | Very sensitive | Dark green spot on light green plate | Sensitivity can be enhanced by use of cobalt(II) chloride |
Ehrlich’s Reagent (Dimethylaminobenzaldehyde) | Indoles, amines | Pink or red-violet | |
Dragendorff-Munier Stain | Amines even the ones that are low in reactivity | Various colors |
In either way, the spots on the TLC plate should be circled (marked) to have a permanent record how far the compound traveled on the plate. Asketch of the developed plate should be placed in your lab notebook. A picture (cell phone) could not hurt either.
Analysis
The components, visible as separated spots, are identified by comparing the distances they have traveled with those of the known reference materials. Measure the distance of the start line to the solvent front (=d). Then measure the distance of center of the spot to the start line (=a). Divide the distance the solvent moved by the distance the individual spot moved. The resulting ratio is called Rf-value. The value should be between 0.0 (spot did not moved from starting line) and 1.0 (spot moved with solvent front) and is unitless.
The Rf (=retardation factor) depends on the following parameters:
- solvent system
- absorbent (grain size, water content, thickness)
- amount of material spotted
- temperature
Due to the fact that all those variables are difficult to keep constant, a reference compound is usually applied to the plate as well.
Tlc Plate Drawing Tool Online
Useful links